The Zimmer Lab

Prof. Manuel Zimmer
Vice Head of Department of Neurosciences and Developmental Biology
Professor for Neural Network Dynamics and Behavior
In the Zimmer lab, we are interested in how neural network dynamics in the brain represent sensory information and perform computations to generate decisions and subsequent behaviors. Moreover, we aim to explain fundamental properties of neuronal circuits, for example the need to sleep.
These are key problems in neuroscience, each of which have alone challenged worldwide communities of experts for decades. We, however, propose that a holistic approach should be undertaken to understand these functions in their full context. To make this goal achievable, we take advantage of the uniquely experimentally tractable model organism C. elegans, a 1mm long nematode worm that can be found dwelling in soil. C. elegans has a small nervous system of only 302 neurons with a completely mapped connectivity map. Nevertheless, it can produce sophisticated behaviors. In recent years, we developed new approaches to quantify C. elegans behavior in unprecedented detail and to record the activity of all neurons simultaneously in real time. These new technologies, together with the rich and efficient genetic toolkit available for the worm, will allow the first complete understanding of any nervous system’s operational principles. In the long term our holistic approach will enable us to generate a realistic in silico simulation of the brain’s properties and behaviors. We will provide a basic proof of principle working model to guide the study of higher nervous systems and the design of brain inspired computational devices.
Research Interests
-
Neuronal population dynamics in C. elegans orchestrate hierarchies of motor actions
We developed a nuclear localized calcium indicator protein that together with fast volumetric fluorescence microscopy enabled us to make true a long-standing dream: to measure brain wide activity in real time and at single cell resolution (Movie, Figure 1A) (Schrödel et al., 2013, Nature Methods). A milestone discovery obtained from such recordings was that most of the active neurons in the awake brain coordinate amongst each other to form collective global activity patterns, even in the absence of any sensory stimulus. Brain states with characteristic neuronal activity pattern are repeated in a cyclical fashion. This can be visualized with computational methods revealing features characteristic of dynamical systems (Figure 1A-B) (Kato et al., 2015, Cell). A thorough investigation of these neuronal population dynamics showed that they correspond to action commands and their assembly into a behavioral command sequence (forward-crawling, backward crawling, turning; Figure 1B) i.e., worms engage in fictive behavioral patterns under such conditions (Kato et al., 2015, Cell).
These basic locomotor states, however, are not the only behavioral representations that can be found in our datasets: investigating the activities of motor neurons we discovered a central pattern generator circuit that operates on a faster time-scale, and which controls explorative movements of the head and nose, termed head casts. Intrigued by these findings, we investigated these behaviors and thereby discovered a hierarchy of movement patterns that is reflected by a hierarchy of neuronal dynamics in the brain (Kaplan et al., 2020, Neuron) (Figure 2).
Our findings impact the current understanding of the principles of neural network function. First, neural networks are not simple input-output devices but exhibit rich intrinsic dynamical properties that interact on par with external inputs. Second, the high-level and long-timescale organization of behavior i.e., the assembly of action sequences, is represented globally at the network level. Activities of smaller groups of neurons that control faster time-scale movement patterns are hierarchically nested within these global network states. This uncovered for the first time a neuronal correlate for a behavioral hierarchy, a neuro-ethological principle postulated decades ago by the ethologists Tinbergen and Dawkins.
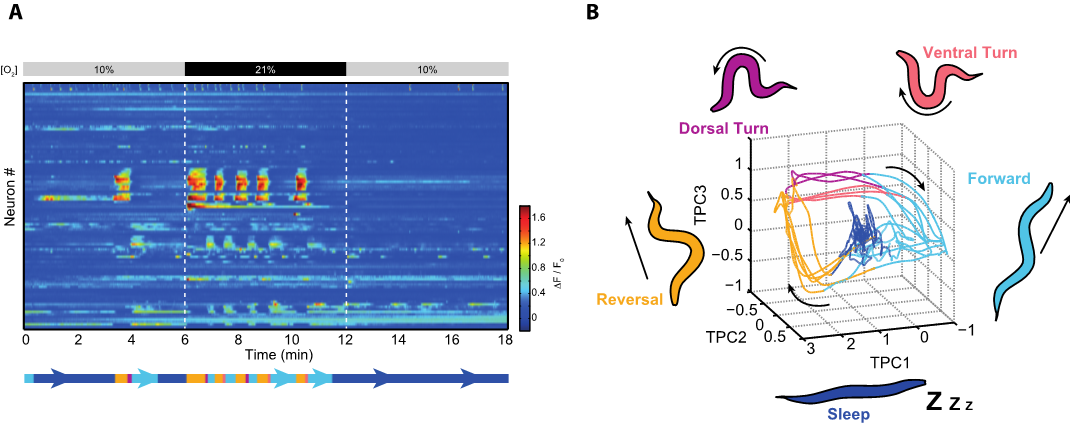
Figure 1. (A) Heatmap from the recording shown in the Movie. Each row represents the activity of a neuron over time; rows are sorted by correlation. Colors indicate relative change in fluorescence intensities. The worm is aroused by an atmospheric (21%) oxygen stimulus as indicated. During phases of arousal an action command sequence can be decoded from these neuronal activities. In the remaining time the brain resides mostly in a sleep state. (B) Time evolution of the brain state from the above recording represented by a phase plot in principal components space. Brain activity evolves on a recurrent attractor cycle that corresponds to the action command sequence (forward crawling – reverse crawling – dorsal vs. ventral turn; also shown as an ethogram below the heatmap). Behavior related brain activity corresponds to phasic attractor states on the cycle during which neuronal activities are dynamically changing (Kato et al., 2015, Cell). This is unlike sleep, which corresponds to a fixed-point attractor during which many neurons are downregulated exempting the sustained activity of sleep-active neurons (Nichols et al., 2017, Science) 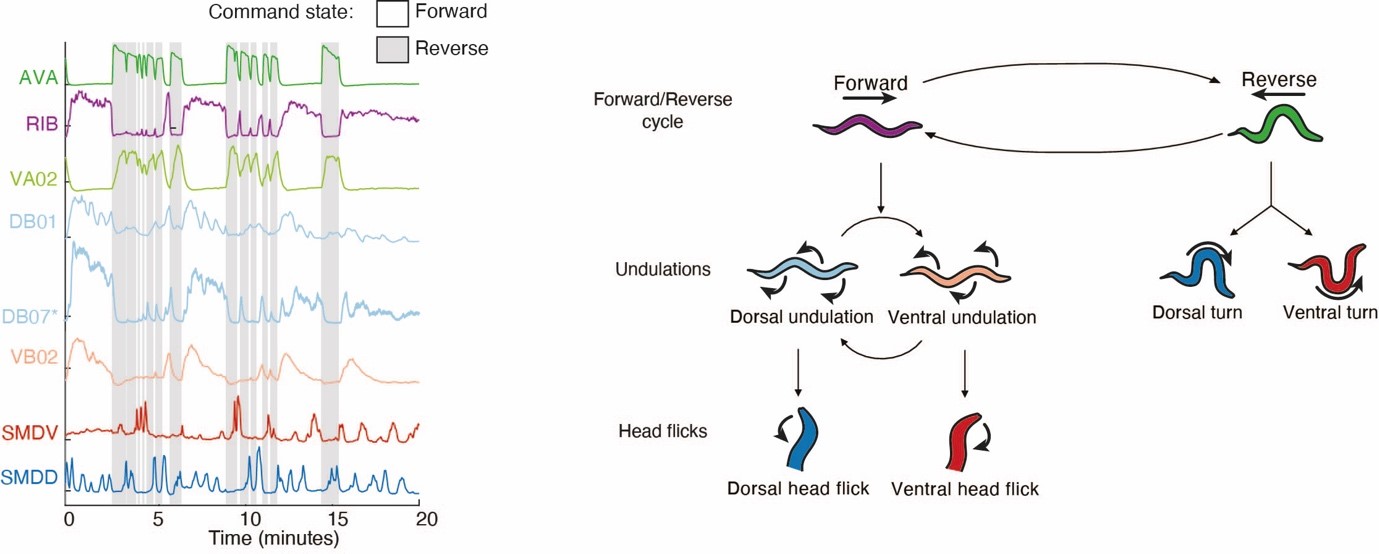
Figure 2. Left panel: example neuronal activity traces from whole brain Ca2+-imaging experiments (neuron types are indicated on the left). AVA and RIB neurons are shown as representatives of backward- and forward-crawling command interneuron populations respectively. VA02 is a backward crawling motor neuron. DB01, DB07 and VB02 are forward crawling motor neurons. SMDV and SMDD constitute a central pattern generator circuit for head-casting movements (head flicks). These nested neuronal activity patterns establish a behavioral hierarchy shown on the right panel (See Kaplan et al., 2022, Neuron, for details). -
From connectome to function: connectivity features underlying neuronal population dynamics
The wiring architecture of neuronal networks is assumed to be a strong determinant of their dynamical computations. An ongoing effort in neuroscience is therefore to generate comprehensive synapse-resolution connectomes alongside brain-wide activity maps. However, the structure-function relationship, i.e. how the anatomical connectome and neuronal dynamics relate to each other on a global scale remains unsolved. To address this, we systematically compared graph features in the C. elegans connectome with correlations in nervous system wide neuronal dynamics obtained from whole brain Ca2+-imaging (Figure 3, upper panels). We found that direct connectivity is a weak predictor of neuronal interactions. However, non-local connectivity features such as specific triplet motifs or similarity measures in network neighborhoods (e.g., input similarities) correlate well with functional interactions between neurons (Figure 3, lower panels). Surprisingly, quantities such as connection strength or the amount of common inputs do not improve these predictions, suggesting that the network’s topology is sufficient. We demonstrate that highly interconnected hub neurons (termed rich club neurons) in the connectome are key to these relevant graph features. Consistently, inhibition of multiple hub neurons specifically disrupts brain-wide activity dynamics. Thus, we propose that the rich club hub-architecture and non-local connectivity features provide an anatomical substrate for coordinated global brain dynamics and that neuronal communication within circuits cannot be fully understood when studied in isolation but within the full context of the connectome (Uzel et al., 2022, Current Biology).
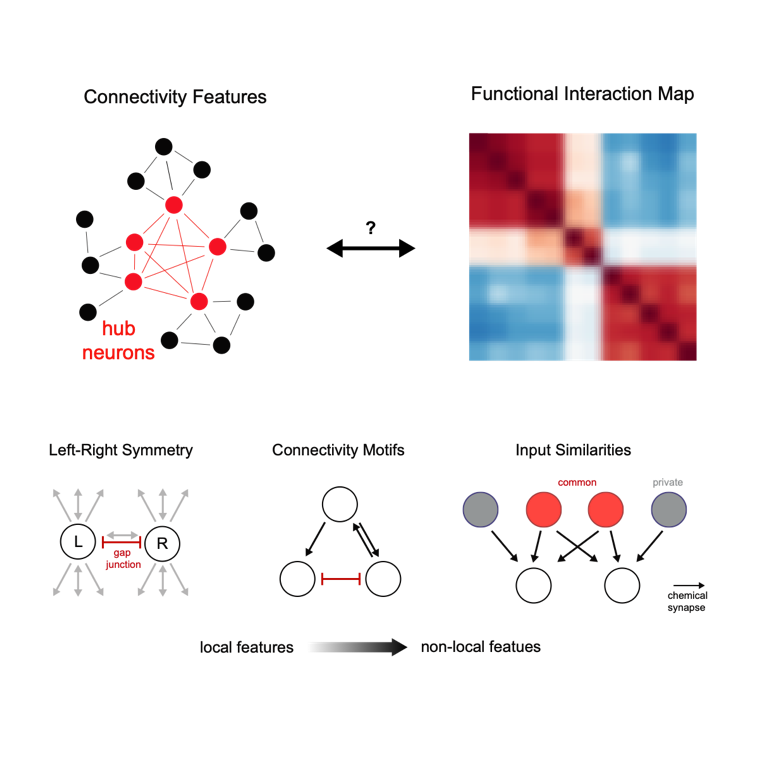
Figure 3. We compared the connectome (upper left) with brain interaction maps (upper right); both simplified schematics. Functional interactions between neurons are supported by GAP junctions but are best understood by connectome context, like triplet motifs and input similarities, rather than by direct connectivity (lower panels). The rich hub architecture (schematized in upper rihght panel) is crucial for establishing this context. Adapted from (Uzel, 2022, Current Biology) -
Functions of brain wide motor representations
What are the functions of brain wide behavior representations? An intriguing feature of them is that they encompass not only motor circuits but also neurons previously thought to exclusively process sensory information. Importantly, such observations were recently made across species, like fruit flies and mice (reviewed in Kaplan et al., 2020, Current Opinion in Neurobiology). One of the major current research goals in the lab is to elucidate the functions of this newly emerging neurobiological principle. In one of our pioneer studies in these directions, we made the surprising observation that even primary sensory neurons retrieve information about the current behavioral state: the BAG carbon dioxide and oxygen sensory neurons are temporally inhibited during short backward crawling episodes. This so termed ‘corollary discharge’ signal is transmitted from the RIM reversal interneuron via extra synaptic tyramine signaling to a cognate ligand gated chloride channel (LGC-55) expressed in BAG (Figure 4). Interestingly, worms can smell their own self-produced carbon dioxide plume when crawling backward into their previous path (Figure 4A). We found that transient inhibition serves the function to reduce perception of this self-induced stimulus (reafference cancelation), which improves perception of external carbon dioxide in the environment (Riedl et al., 2022, Current Biology). We thus propose that reafference cancellation is one of the perhaps multiple functions of brain wide motor representations.
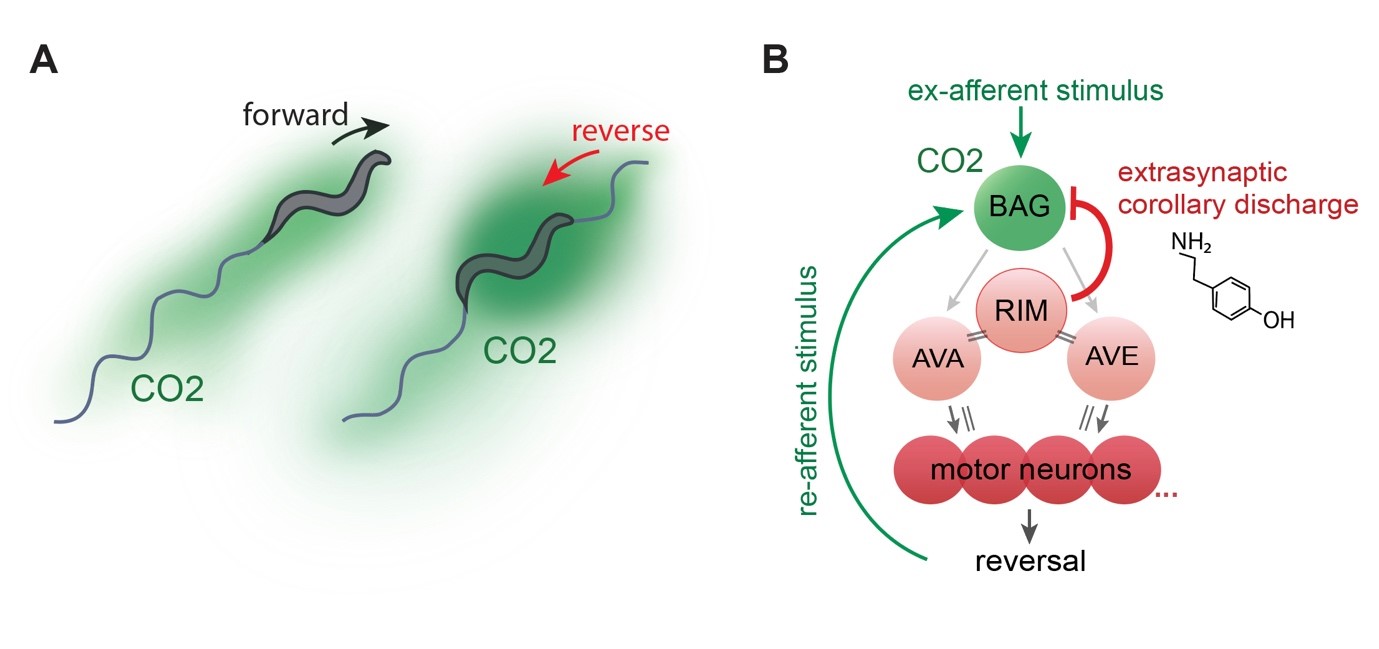
Figure 4. (A) Sketched distribution of CO2 plumes (green) around a forward crawling (left) and reversing animals (right), which experiences elevated CO2 levels. (B) BAG neurons connect to a network of reversal command neurons via its postsynaptic partners AVA and AVE (for simplicity only RIM is shown in addition), which in turn trigger reversal events by activating the reversal motor neuron pool (only 4 neurons shown for simplicity). During these network states, RIM neurons convey a tyraminergic corollary discharge signal to LGC-55 expressed in BAG. This mechanism filters re-afferent perception of a CO2 stimulus caused by reversal movement as shown in (A). Grey arrows, chemical synapses; double lines, gap junctions. From (Riedl et al., 2022, Current Biology) -
Control of sleep and wakefulness
We developed a new paradigm to effectively switch between wakefulness and sleep during whole brain recordings. Animals are alerted and aroused when exposed to atmospheric oxygen levels as they prefer intermediate oxygen concentrations found in their natural habitats. During developmentally timed sleep-prone episodes termed lethargus, they exhibit sleep behavior at intermediate oxygen levels but can be effectively kept awake by atmospheric oxygen (Figure 1A-B). We found that sleep is a state of brain wide quiescence exempting a set of sleep active neurons. While behaviors in awake animals are represented by dynamic network activity as described above, sleep appears as a stationary fixed point in neuronal state space (Figure 1B). When worms are left unperturbed this sleep state spontaneously emerges, but can be rapidly interrupted by the arousing oxygen stimulus. Our work demonstrates for the first time a global activity signature of sleep in an invertebrate, and it is the first brain-wide single cell resolution sleep study in any animal. Our results support the hypothesis stating that sleep arises via a self-organizing network mechanism; however, the brain exerts control over its global state via sleep active neurons and opposing arousal circuits (Nichols et al., 2017, Science).

Figure 1. (A) Heatmap from the recording shown in the Movie. Each row represents the activity of a neuron over time; rows are sorted by correlation. Colors indicate relative change in fluorescence intensities. The worm is aroused by an atmospheric (21%) oxygen stimulus as indicated. During phases of arousal an action command sequence can be decoded from these neuronal activities. In the remaining time the brain resides mostly in a sleep state. (B) Time evolution of the brain state from the above recording represented by a phase plot in principal components space. Brain activity evolves on a recurrent attractor cycle that corresponds to the action command sequence (forward crawling – reverse crawling – dorsal vs. ventral turn; also shown as an ethogram below the heatmap). Behavior related brain activity corresponds to phasic attractor states on the cycle during which neuronal activities are dynamically changing (Kato et al., 2015, Cell). This is unlike sleep, which corresponds to a fixed-point attractor during which many neurons are downregulated exempting the sustained activity of sleep-active neurons (Nichols et al., 2017, Science) -
Quantitative behavior
In order to understand brain functions we aim for a comprehensive quantitative description of its behavioral outputs. We developed high throughput behavioral recording techniques to measure the crawling movements of worms in terms of detailed posture time series (Figure 5). Using a computational method based on principal components analysis, we showed that behavior can be composed out of two elementary motor patterns: the undulation mode contributes to the stereotyped sinusoidal crawling pattern of the animals, while the turning mode contributes to steering and reorientation movements (Figure 5). We identified peptidergic interneurons (termed AVK and DVA) that can gradually control both motor patterns to achieve a spectrum of different behaviors. This work suggests that animals can flexibly generate behavioral diversity out of simple elementary movement modes (Hums et al., 2016, eLife).
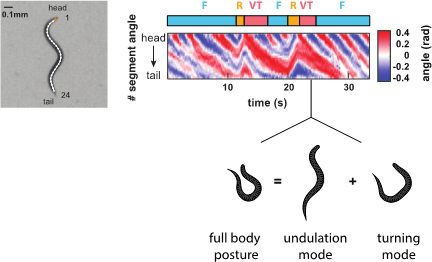
Figure 5. Behavioral analysis of C. elegans. (Left) Worms are filmed and their images skeletonized. 24 inter-segment angles are used to describe each posture. (Right) A posture time series of two consecutive action sequences as described in the ethogram above (Forward crawling – Reverse crawling – Ventral Turn). Shown below is a worm silhouette during a ventral turn. Its complex posture can be decomposed into two elementary postures termed undulation mode and turning mode. Our work shows that worms can adopt many intermediates between such extreme postures by varying the relative contribution of each elementary mode
Current and Future Research
In our current and future work, we combine advanced microscopy techniques for whole brain and whole nervous system imaging with quantitative behavior, molecular genetics and computational neuroscience. Our research objectives remain focused on brain dynamics crucial for nervous system function. We aim to understand the information flow from sensory inputs via decision making to behavioral output, and why these processing stages occur in such a distributed (as opposed to sequential) manner. We also aim to solve why such a system requires a sleep-wake cycle. Finally, our findings will be recapitulated in a realistic in silico simulation of brain dynamics and behavior. This holistic approach will enable us for the first time to understand the operational principles of the worm’s nervous system, which we propose are generalizable to larger animals. We are focusing on a few major objectives:
- To develop new light-sheet microscopy approaches for whole nervous system imaging in freely behaving worms.
- Using whole brain imaging combined with complex sensory stimulation protocols, we are studying how sensory circuits represent features of the environment and interact with brain dynamics to control behavior. This approach will uncover the neural basis of computations and decision making as well as all functions of brain wide motor representations.
- Using graph theory, we are analyzing the worm’s connectome to inform targeted circuit manipulations of its nervous system. Here we aim to further uncover the crucial features in anatomical wiring architecture that serve neuronal function.
- While neuronal population dynamics in worms represent a global framework for long timescale action sequences, it remains to be addressed how these action commands are transformed into individual actions and movement patterns at the motor periphery.
- How are whole brain dynamics affected by sleep deprivation and subsequent rebound sleep? Studying sleep homeostasis will shed light on a major mystery: why do animals need to sleep?
- We will reverse engineer the brain in a faithful comprehensive network simulation; the first one available for any complete nervous system. In collaboration with computer scientists, we will leverage this computational platform to design novel brain inspired robotics algorithms and devices.


