Advancing a powerful marine model system
Neural regeneration is likely an ancestral feature secondarily lost in mammals and other taxa. Annelid worms modulate regenerative capacity through brain-derived factors. These also orchestrate other developmental features – ranging from bristle shapes to reproductive maturation – and even the time of death. Research into such factors therefore provides unique experimental and mechanistic access to fascinating and fundamental aspects of biology.
Our research combines functional work (knock-outs, transgenics), cell biology, multimodal imaging, cellular profiling (scRNAseq), and physiological and behavioral strategies. Most of our work focuses on the marine bristleworm Platynereis dumerilii that we have helped to push as an experimental system. Our efforts in exploring the bristleworm as a unique system for stem cell biology are embedded in larger Vienna-wide efforts to address the molecular mechanisms of stem cell modulation, differentiation and regeneration.

The marine bristleworm Platynereis dumerilii, a highly versatile and powerful laboratory model system.
Exploring the natural "reprogramming" of cells into stem cells
Stem cells are central for both the development of organisms and their ability to replace lost tissues. Our laboratory bristleworms grow by adding new segments at their posterior ends using a stem cell zone. But what happens if the zone is cut off? Almost 60 years ago, pioneering work by Dietrich Hofmann showed using histology that only hours after such amputations, cells adjacent to the wound undergo “re-embryonisation”, exhibiting enlarged nuclei and nucleoli. This suggested a process modern stem cell biology refers to as “dedifferentiation”. Combining a time-resolved single-cell RNA sequencing approach with high-resolution imaging of markers in the emerging regenerate, we revealed that indeed, stem cell signatures emerge in differentiated cells, at the expense of differentiation markers. Bristleworms thus possess a natural system to reprogram differentiated cells into stem cells. Our detailed characterization of this process also allows us to explore how this process is orchestrated by hormonal cues
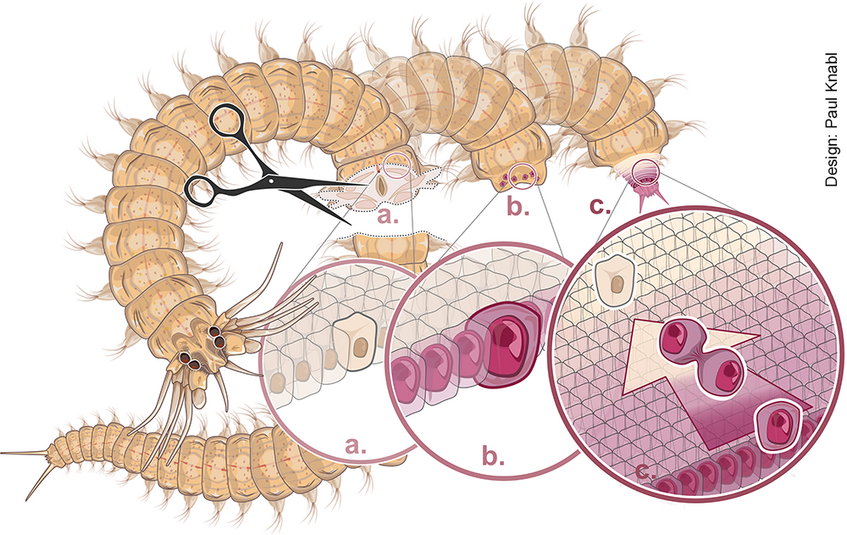
When worms lose their tail (a), differentiated cells close to the wound are molecularly re-programmed into new stem cells (b), and start to divide to build up the missing segments (c). See Stockinger, Adelmann et al., Nat. Comms. 2024. doi.org/10.1038/s41467-024-54041-3
A unique chance to explore biomaterials and their production
Our bristleworm model also provides unique access to the mechanisms involved in biomaterial production. One of the fascinating aspects of bristleworms is the generation of stereotypical bristles with nanometric precision. In a recent, collaborative study, we revealed that the underlying process exhibits striking similarities to the “material jetting” approach of technical 3D printing. Dedicated cells “eject” chitin polymers into the extracellular space, while changing their cell surface over time in a highly stereotypical fashion. In combination, this appears to enable the precise sculpting of chitin into stereotypical micro- to submicrometric morphological features. Our experiments provide a highly interesting entry point into exploring the molecular and cellular mechanisms underlying such nanoscopic morphologies. The bristleworms also feature additional, intriguing materials such as a special biological matrix present in the animal’s jaws. Our molecular advances make it possible to decode fundamental principles of such materials and their natural production, with significant promise for bioengineering.
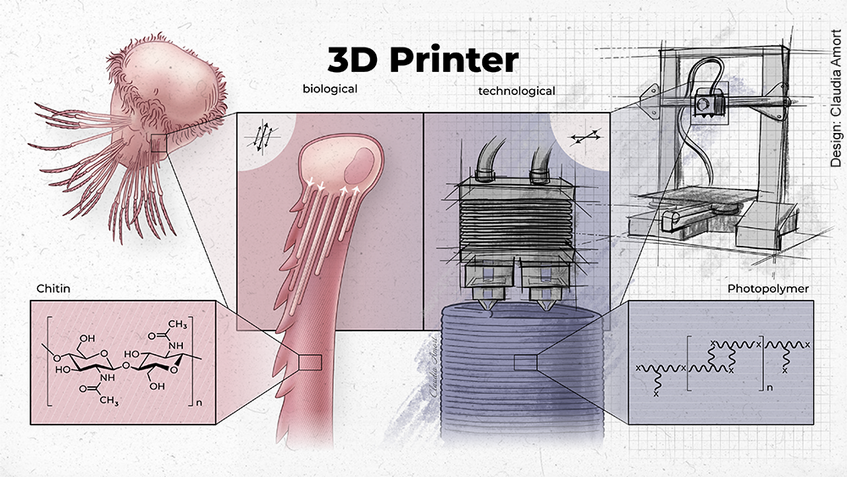
The formation of chitin bristles relies on a “biological 3D printing” mechanism encoded by individual chaetoblast cells. In a material-jetting-like process chitin polymers are ejected into the extracellular space, allowing for the manufacturing of detailed, subm-micrometric features. See Ikeda et al., Nature Comms. 2024: doi.org/10.1038/s41467-024-48044-3